Unlock the power of your chromatography data at enterprise-scale

Performance Trending
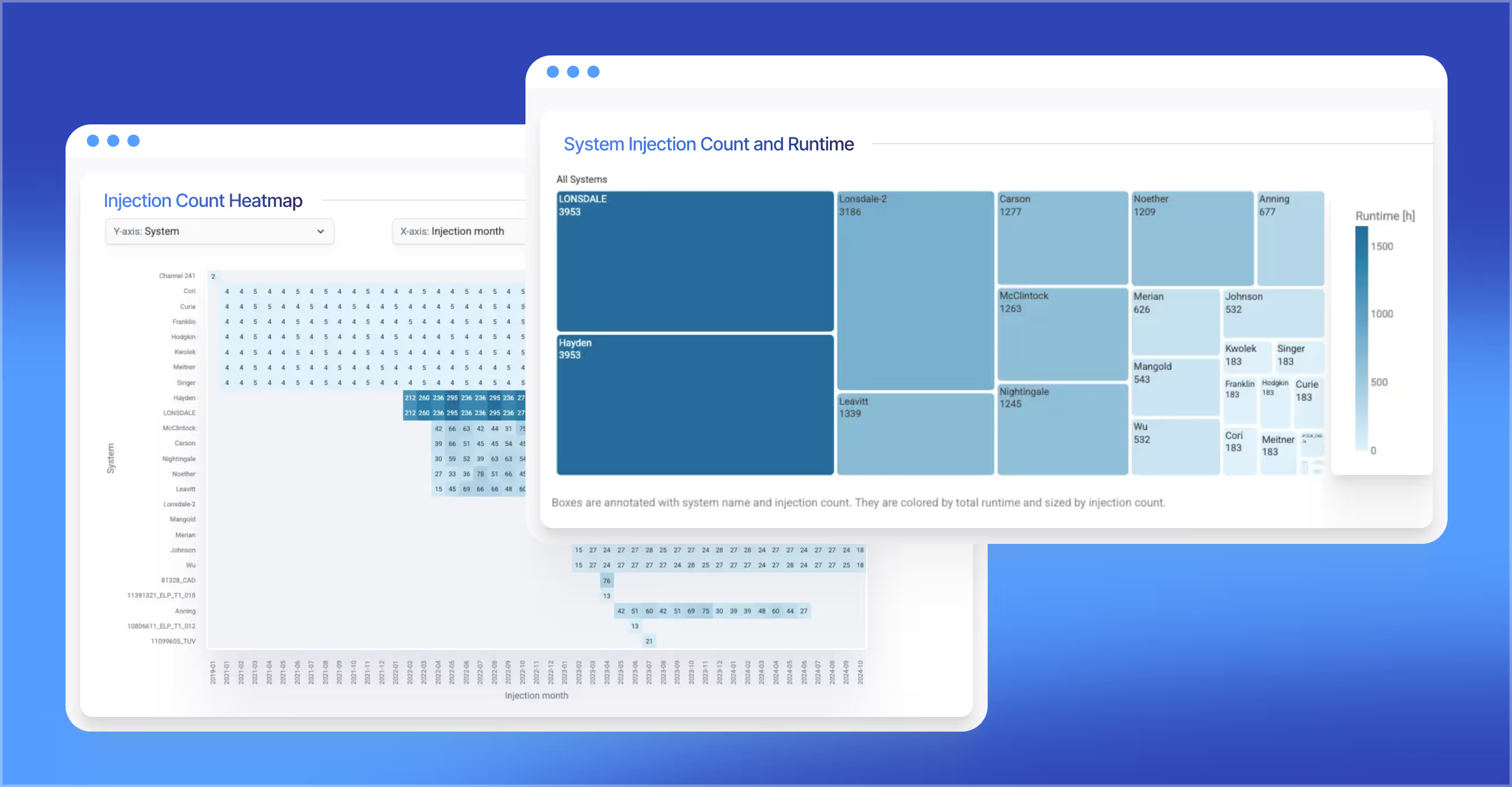
Visually compare chromatographic runs to quickly identify method performance changes. The new Chromatogram Overlay feature turns days of numerical analysis into minutes of visual troubleshooting, accelerating method development and optimization.

Column Logbook
NEW! Digitally track the complete lifecycle of every column—from first use to retirement. Create traceability reports in minutes instead of days, replacing physical logbooks with comprehensive digital records for enhanced cGMP compliance.
Key benefits and business outcomes
Reduce out-of-spec events up to 75%
Proactively monitor data trends across projects, departments, and sites—not possible today—for early detection.
Increase productivity and data integrity by at least 50%
Instantly identify method performance issues across instruments, columns, and reagents without switching between vendor-specific tools or manual data aggregation.
Improve compliance and reduce risk
Surface critical compliance data points—such as injection counts, sample duplicates, and result ratios—to streamline audits and minimize risk.
Lay the groundwork for predictive insights and AI
Turn historical and recent data into robust AI models to optimize method development, forecast outcomes, and auto-detect anomalies.
What can Chromatography Insights do for you?
Accelerate method development with accessibility to historical and recent data across projects, departments, and users
Ensure smooth tech transfers with proactive titer and purity data monitoring across development and QC sites and CDMOs
Cut troubleshooting time from days to hours with complete visibility across batches and sites
Build better methods faster using historical data patterns
Proactively monitor product quality within and across batches of drug substances and products for better process control
Improve batch consistency and release by tracking impurity trends along with system suitability and control samples
Surface and flag anomalies early that may trigger deviations and costly investigations
Enhance compliance and streamline audits with full visibility into critical compliance data points
Get FAIR, AI-ready chromatography data faster with prebuilt pipelines that cut data preparation time by 50%—centralized and harmonized across CDSs, projects, and sites
Eliminate duplication and governance overhead with curated datasets accessible directly into your tool of choice like Databricks or Snowflake
Enable reliable insights and scalable models by building on complete, accurate datasets with traceable lineage
Accelerate decisions with an enterprise-ready dashboard that surfaces hidden patterns, method variability, and anomalies across chromatography data
Accelerate method development with accessibility to historical and recent data across projects, departments, and users
Ensure smooth tech transfers with proactive titer and purity data monitoring across development and QC sites and CDMOs
Cut troubleshooting time from days to hours with complete visibility across batches and sites
Build better methods faster using historical data patterns
Proactively monitor product quality within and across batches of drug substances and products for better process control
Improve batch consistency and release by tracking impurity trends along with system suitability and control samples
Surface and flag anomalies early that may trigger deviations and costly investigations
Enhance compliance and streamline audits with full visibility into critical compliance data points
Get FAIR, AI-ready chromatography data faster with prebuilt pipelines that cut data preparation time by 50%—centralized and harmonized across CDSs, projects, and sites
Eliminate duplication and governance overhead with curated datasets accessible directly into your tool of choice like Databricks or Snowflake
Enable reliable insights and scalable models by building on complete, accurate datasets with traceable lineage
Accelerate decisions with an enterprise-ready dashboard that surfaces hidden patterns, method variability, and anomalies across chromatography data